1. What is GMP?
GMP (Good Manufacturing Practice) refers to the regulations on Good Manufacturing Practice issued by the U.S. Food and Drug Administration (FDA).
The primary goal of GMP standards is to minimize the risk of contamination by microorganisms, particles, and pyrogens during the preparation and sterilization of medicines or medical devices. Any application related to the production, filling, mixing, or packaging of sterile pharmaceutical products must adhere to GMP standards to ensure the safety and consistency of the products.

GMP controls key production processes such as:
Personal hygiene
Factory premises
Equipment and machinery
Production process control
Sanitation operations
Sanitation measurements
Handling and storage
According to the Ministry of Health, GMP standards are mandatory for all fields of production and processing of products requiring high levels of food safety and hygiene, such as:
Pharmaceuticals
Food industry
Cosmetics industry
Medical devices
Under the Decree 15/2018/ND-CP of the Government detailing the implementation of the Food Safety Law, from July 1, 2019, all production facilities for health protection products and dietary supplements must meet GMP standards.
EU GMP Standards
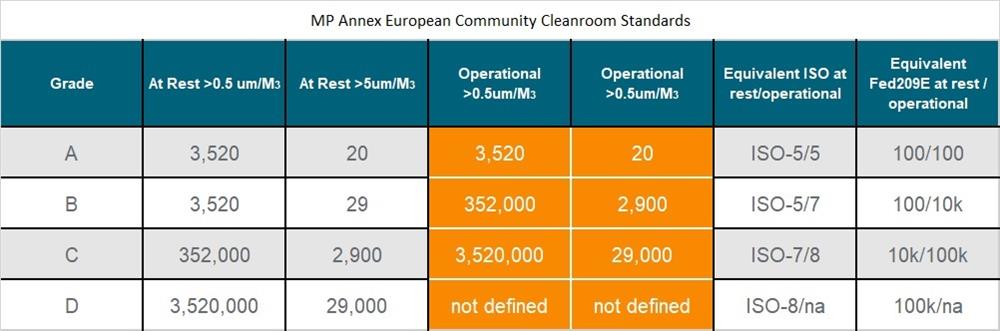
2. What is GMP Cleanroom?
A GMP cleanroom is a room that combines technical design, manufacturing, finishing, and control operations necessary to meet GMP standards.
However, the requirements in GMP principles are established flexibly and broadly, allowing each manufacturer to operate in the best way to implement these standards. Depending on the field, size, and financial conditions, manufacturers can decide on the number of regulations, standards, procedures, and work instructions to meet the necessary requirements. Therefore, the procedures in the GMP system at each facility or enterprise will vary, not uniform.
Pharmaceutical Cleanroom Air Systems
3. Types of GMP Certifications and Issuing Authorities:
WHO GMP Certification is issued by the Drug Administration.
EU GMP Certification is issued by national drug regulatory agencies or regional drug regulatory agencies in EU member states.
PIC/S GMP Certification is issued by competent pharmaceutical regulatory bodies in PIC/S member countries.
HS GMP Certification is issued by the Food Safety Department.
ASEAN GMP Certification is issued by the Drug Administration.
Japan-GMP Certification is directly issued by the Pharmaceuticals and Medical Devices Agency (PMDA) of Japan.
Construction of GMP-Compliant Cleanrooms
Japan-GMP is the Good Manufacturing Practice standard for pharmaceuticals in Japan. It was established in Japan in 1974 and came into effect in 1975, before EU-GMP (1989), and has been continuously updated to increase its rigor. Japan-GMP represents Japan's quality standards. This pharmaceutical manufacturing practice establishes technical barriers for raw material standards, sterile finished product storage, quality system management under ICH Q10 standards, etc., to ensure that medicines meet the highest quality standards before reaching patients. Japan-GMP is recognized in many countries worldwide, including Vietnam. Japan-GMP is also a standard for medicines in hospitals, used for treating mild to severe conditions.


Steps to Achieve GMP Certification:
Step 1: Prepare and study the necessary documentation:
Current legal regulations.
Standards applicable to the products.
Technical operation requirements.
Feedback from customers and stakeholders about the products.
New scientific information and practical experience from the business.
Results from product research/testing.
Step 2: Determine the scope of GMP standard application.
Step 3: Develop a plan, set a schedule, and assign responsibilities.
Step 4: Create processes and templates to control all stages of the work.
Step 5: Train employees and staff involved in the production process.
Step 6: Implement the system and operate according to the established processes.
Step 7: Address any unresolved or non-compliant issues during GMP implementation.
Step 8: Officially approve and publish the GMP production process.
Step 9: Conduct internal evaluations to improve the system’s efficiency and plan future improvements.
Step 10: Register for GMP certification with appropriate conformity assessment organizations or competent authorities.

GMP Standards
Documents Required to Apply for GMP Certification for a Pharmaceutical Factory:
Application form for "Good Manufacturing Practice" inspection.
A signed and stamped copy of the facility's registration documents: business establishment certificate, business registration certificate, or investment certificate.
Organizational chart and staff structure of the facility.
Training programs and summary reports on Good Manufacturing Practice training at the facility.
Site plan and design of the factory, including:
Overall layout diagram.
Worker pathways.
Material, packaging, semi-finished product, and finished product pathways.
Water supply system for production.
Air supply system for the factory.
Cleanroom levels within the factory.
Waste disposal system.
List of existing equipment in the factory.

GMP Cleanroom Quality Inspection
To obtain GMP certification for a pharmaceutical factory from the Ministry of Health of Vietnam, the pharmaceutical company must follow these steps:
Step 1: Submit the GMP registration file to the Drug Administration – Ministry of Health.
Step 2: The Drug Administration receives, checks, and reviews the file. If the file does not meet the requirements, a request for additional documents will be made.
Step 3: Within 20 working days from the inspection plan notification, the Ministry of Health will conduct an on-site inspection at the registered facility.
Step 4: The competent authority must issue a GMP certificate or officially notify the facility of the results within 5 working days from the end of the inspection (if the requirements are met) or after receiving the facility's corrective action report. If the facility does not meet the GMP requirements, the facility must correct the issues. After completing the self-evaluation and meeting the requirements, the facility can submit the application again from the beginning.
Step 5: Issue the GMP certificate to the registered facility.
For cooperation, please contact us:
BACH LONG TRADE SERVICE MANUFACTURING CO., LTD.
Address: 75 Nguyen Hong Street, Ward 1, Go Vap District, Ho Chi Minh City
Hotline: 093 143 54 54
Email: sales@congnghelockhi.vn
Website: www.congnghelockhi.com - www.congnghelockhi.vn