The modern cleanroom was invented by American physicist Willis Whitfield. He worked at the Sandia National Laboratories and initially conceptualized the cleanroom in 1960.
Three years later, in 1963, the first cleanroom standards were established in the United States. Since then, these standards have been continually improved, evolving into global benchmarks applied across various industries. Cleanrooms are typically classified according to the Federal Standard 209 E, ISO 14644-1, and the Vietnamese standard TCVN 8664-1:2011.

A cleanroom is defined as a controlled environment designed to minimize the entry of particles into the air and to regulate other environmental parameters such as temperature, humidity, and pressure when necessary. By controlling these factors, cleanrooms help prevent contamination or cross-contamination during research and production processes, ensuring sterility and product safety.
Investing in cleanroom construction offers significant potential for profitability and provides a competitive edge. Many industries, particularly those looking to export to large markets, are required to meet specific cleanroom standards. A manufacturing facility with the necessary expertise and cleanroom-based production processes will be prioritized, opening up more business opportunities.
For example, GMP EU standards are critical for pharmaceutical companies seeking to expand into stringent markets like the European Union.
Furthermore, a well-designed cleanroom environment effectively eliminates contaminants that could affect production processes. This stability ensures quality and safety, meeting the required safety standards.
In the future, cleanrooms and cleanroom standards will become mandatory across all business operations. Companies not adhering to these standards must quickly adjust to stay competitive.
Cleanrooms must meet the standards set by the International Organization for Standardization (ISO) to be classified. ISO, established in 1947, develops international standards for sensitive scientific and business practices, such as working with chemicals, volatile materials, and sensitive equipment. ISO's standards are globally recognized, with over 20,000 standards in use today.
Cleanroom standards are technical specifications issued by international standardization organizations to certify the quality of cleanroom design, focusing on cleanliness, humidity, temperature, and pressure. Currently, cleanrooms are classified according to three main standards:
FED STD 209E:
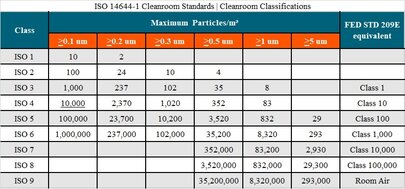
FED STD 209E was once a key cleanroom standard, widely used to classify air cleanliness levels in cleanrooms. However, this standard was replaced by ISO 14644-1 in 2001.

ISO 14644-1
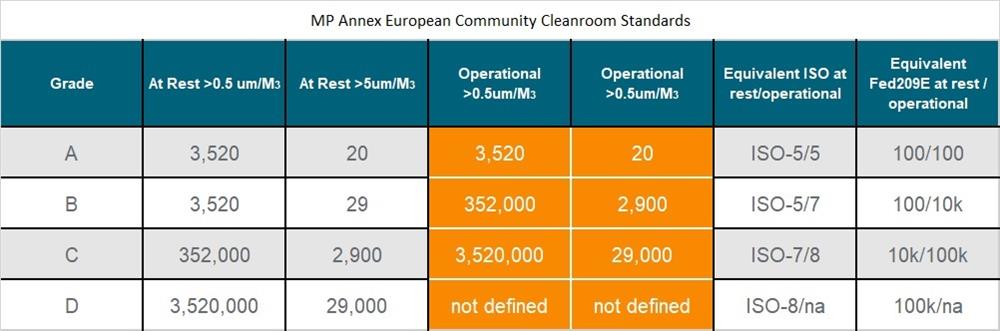
GMP (Good Manufacturing Practice)
Cleanrooms are widely used across several industries, including:
Medical Equipment Manufacturing:
The risk of microbial contamination is high in the production of both disposable and non-disposable medical devices. Failure to manufacture these products in a controlled environment can lead to contamination, posing risks of infections and other health issues for patients. Medical devices like feeding tubes, catheters, and blood tubes must be produced in cleanrooms to prevent contamination and ensure safety.
Pharmaceutical Industry:
Pharmaceutical manufacturing must adhere to strict environmental control conditions, regulated by standards like ISO 14644. Cleanrooms prevent bacterial and particulate contamination in medicines, vaccines, and other biological products. Every stage, from raw material selection to packaging, must comply with stringent protocols.
Cosmetics Manufacturing:
Like pharmaceuticals, cosmetics production also benefits from cleanroom environments. The high levels of bacteria in the production process can lead to degradation of ingredients, causing potential allergic reactions and skin damage. Cleanrooms minimize bacterial contamination, ensuring product quality and safety.
Food Production:
Certain foods, such as milk, fresh pastries, ice cream, and canned products, need to be stored in clean, safe environments. Dust and microbial contamination can cause spoilage and lead to digestive illnesses in consumers. Cleanrooms ensure food safety by reducing the presence of harmful particles during production.
Electronics Manufacturing:
The electronics industry, particularly semiconductor and microchip production, requires the highest standards of cleanliness. Even a single particle of dust can damage sensitive components. Cleanrooms are essential in these industries to ensure air purity, temperature, humidity, and static-free conditions.
Hospital Operating Rooms:
Operating rooms must be sterile to prevent infections. Cleanrooms in hospitals significantly reduce the risk of surgical site infections, ensuring patient safety and faster recovery. They also play a vital role in isolating infectious diseases in quarantine areas.
Factory Cleanrooms:
Cleanrooms are also integrated into factories, either as part of the entire building or in specific areas. The number of cleanrooms depends on the specific requirements of the industry and production processes.
Cleanrooms are also used in research laboratories, in vitro fertilization (IVF) labs, tissue banks, and more, ensuring the preservation of valuable materials and reducing the risk of contamination.
For collaboration, please contact:
Bách Long Trading & Service Manufacturing Co., Ltd.
Address: 75 Nguyen Hong Street, Ward 1, Go Vap District, Ho Chi Minh City
Hotline: 093 143 54 54
Email: info@congnghelockhi.vn
Website: www.congnghelockhi.com - www.congnghelockhi.vn